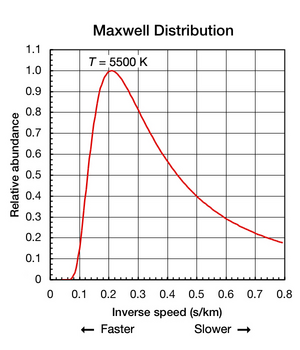
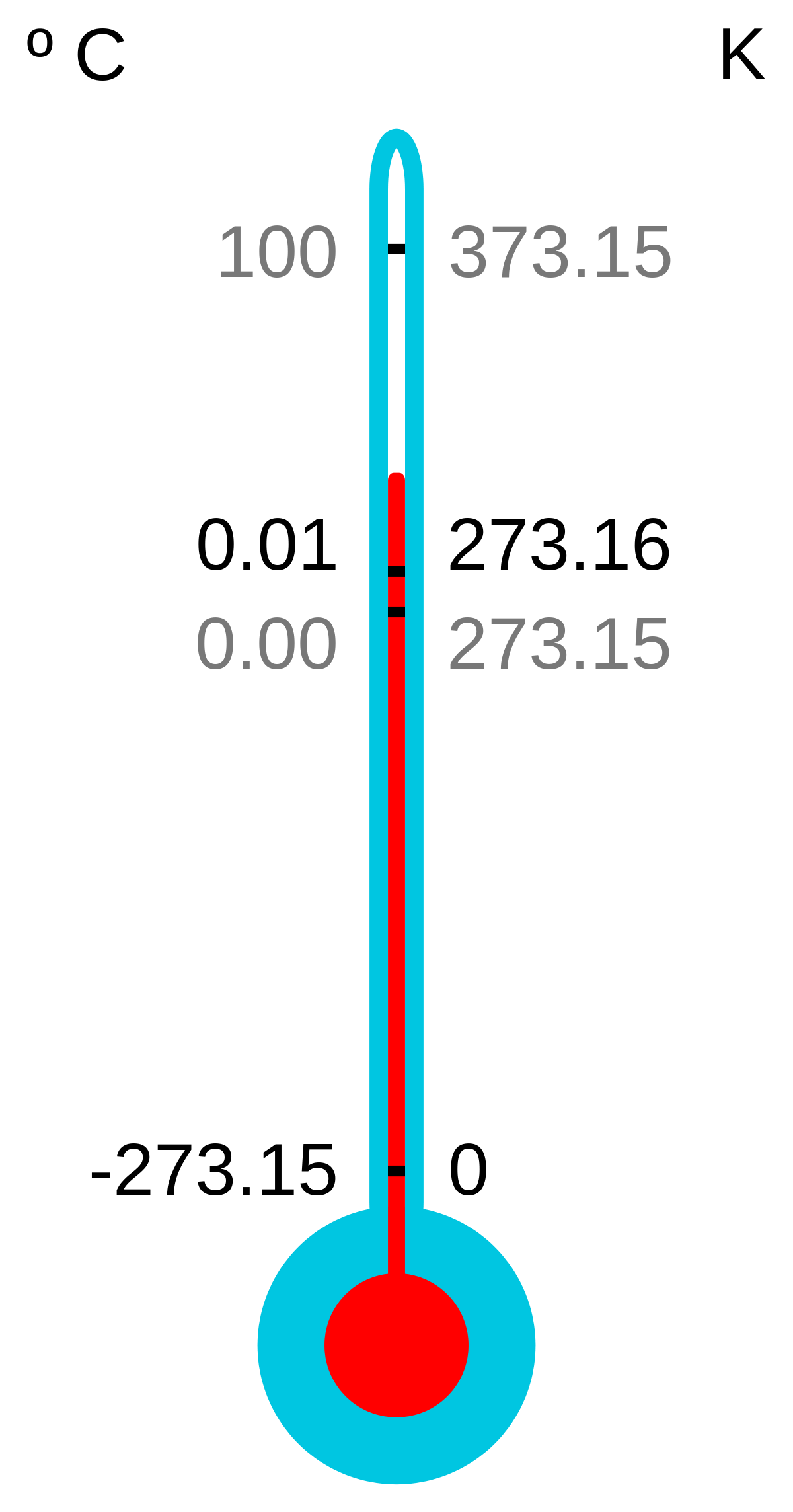
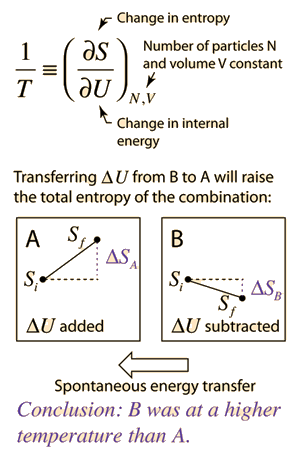
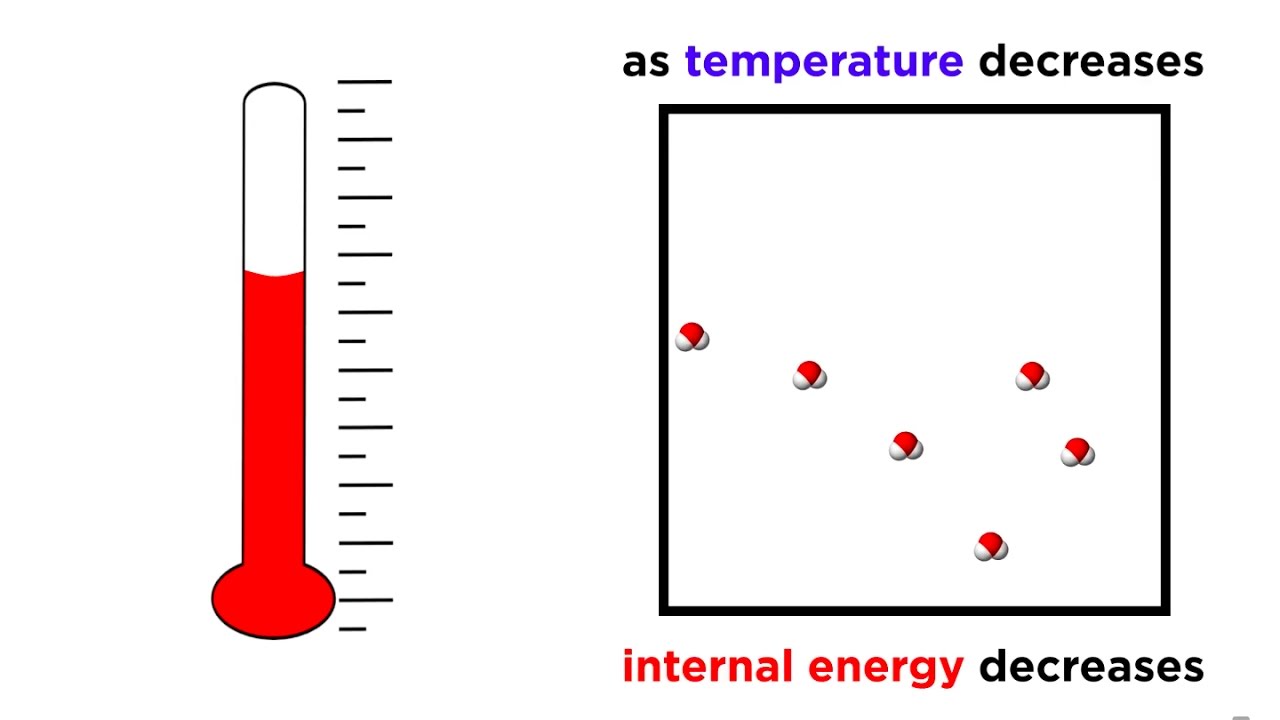
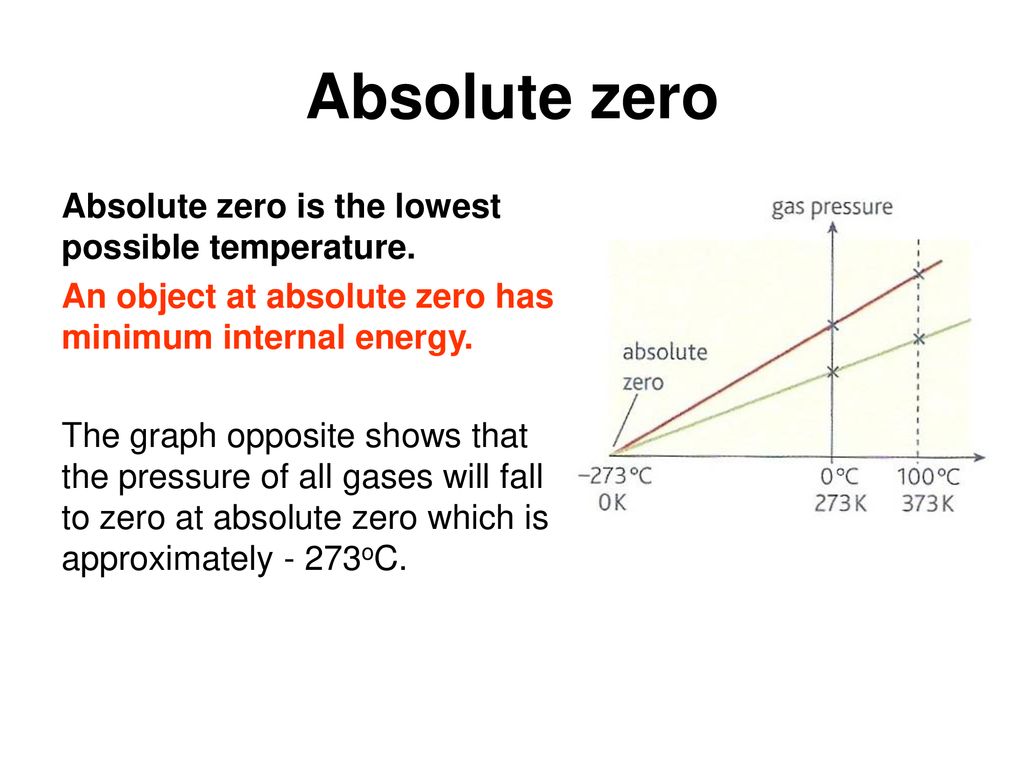
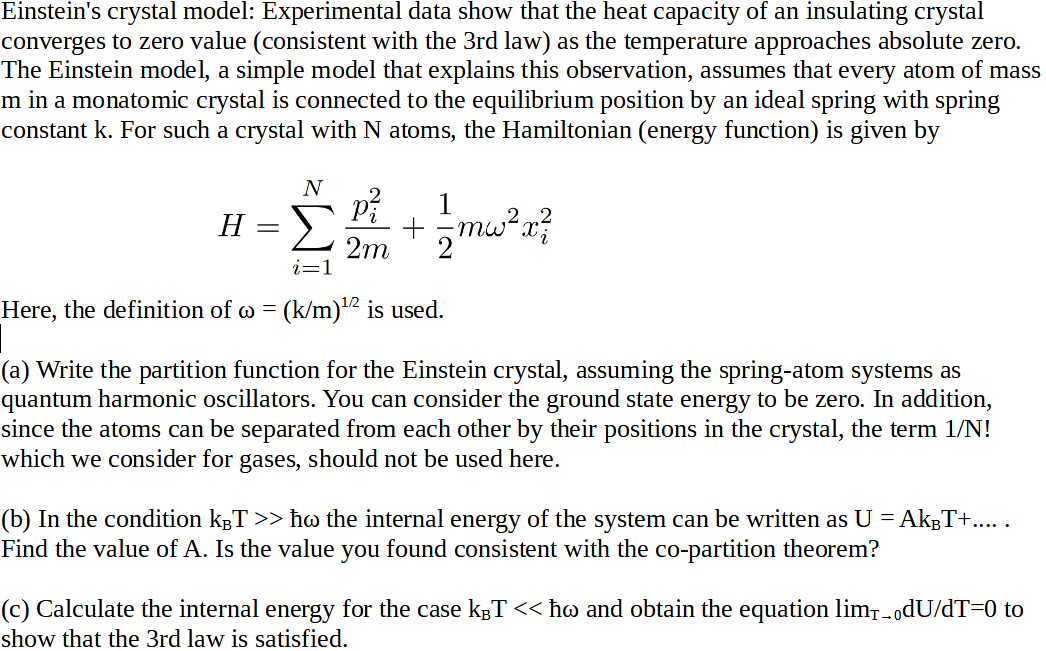
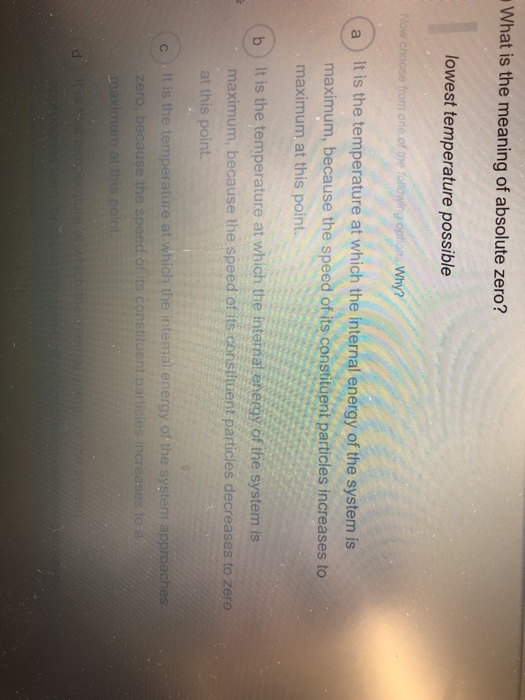
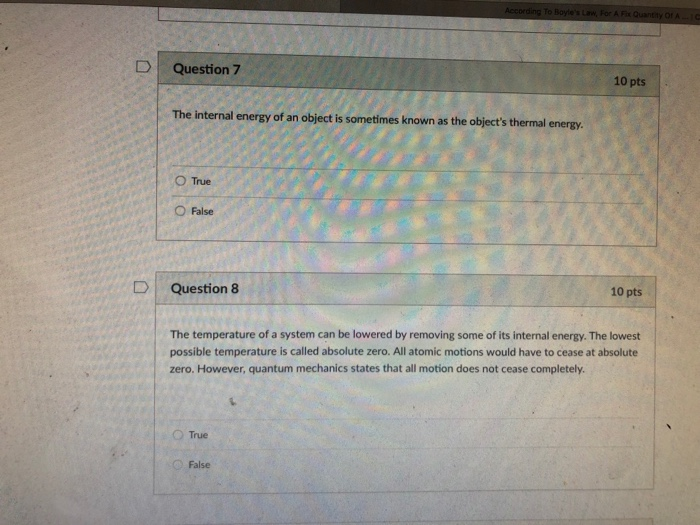
Absolute Zero Objectives (c) describe how there is an absolute scale of temperature that does not depend on the property of any particular substance (ie. - ppt download

The Absolute Zero of Internal Energy and Entropy, and the Corresponding Inertness of Matter | Science

Assertion : Absolute zero is a theoretically possible temperature at which the volume of the gas becomes zero.Reason : The total kinetic energy of the molecule is zero at this temperature.
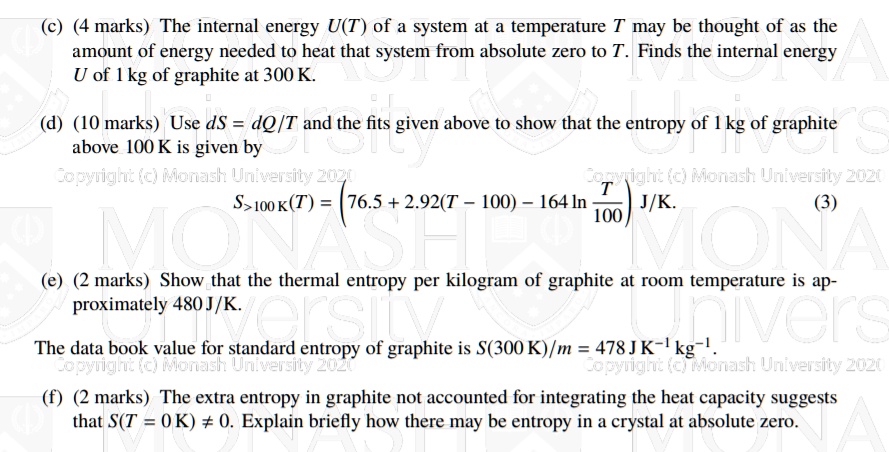
SOLVED: (4 marks) The internal energy U(T, of a system at a temperature T may be thought of as the amount of energy needed to heat that system from absolute zero to
A gas mixture of 3 mole of hydrogen and 2 mole of helium at absolute temp T. Considering all vibrational modes ( assume zero internal energy at T=0 ) the total internal