UNAIDS welcomes the approval of long-acting injectable cabotegravir as a pre-exposure prophylaxis for HIV prevention | UNAIDS

Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study - The Lancet

Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial - The Lancet HIV

Discussion Paper: Research Priorities for Implementing Long-acting Injectable Cabotegravir for PrEP in Australia

Hydrogel-forming microarray patches with cyclodextrin drug reservoirs for long-acting delivery of poorly soluble cabotegravir sodium for HIV Pre-Exposure Prophylaxis - Pharma Excipients
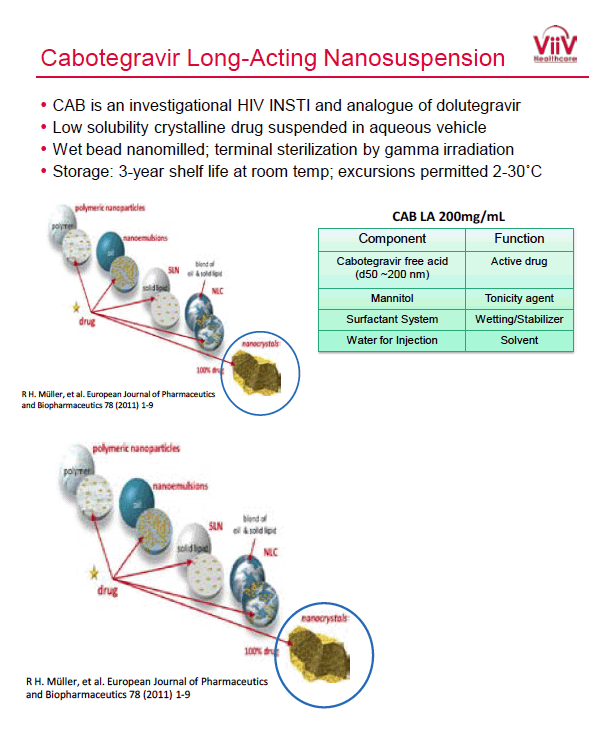
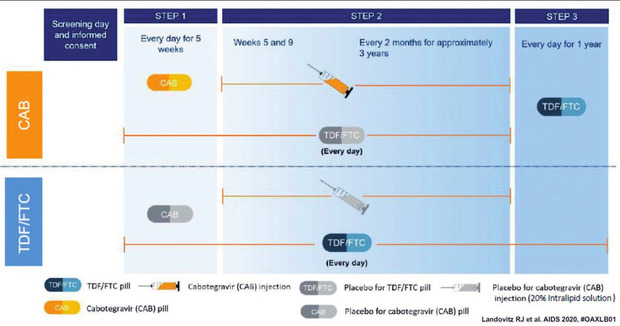
Tremendously exciting” news of cabotegravir long-acting injectable for PrEP shared at AIDS 2020 - San Francisco AIDS Foundation
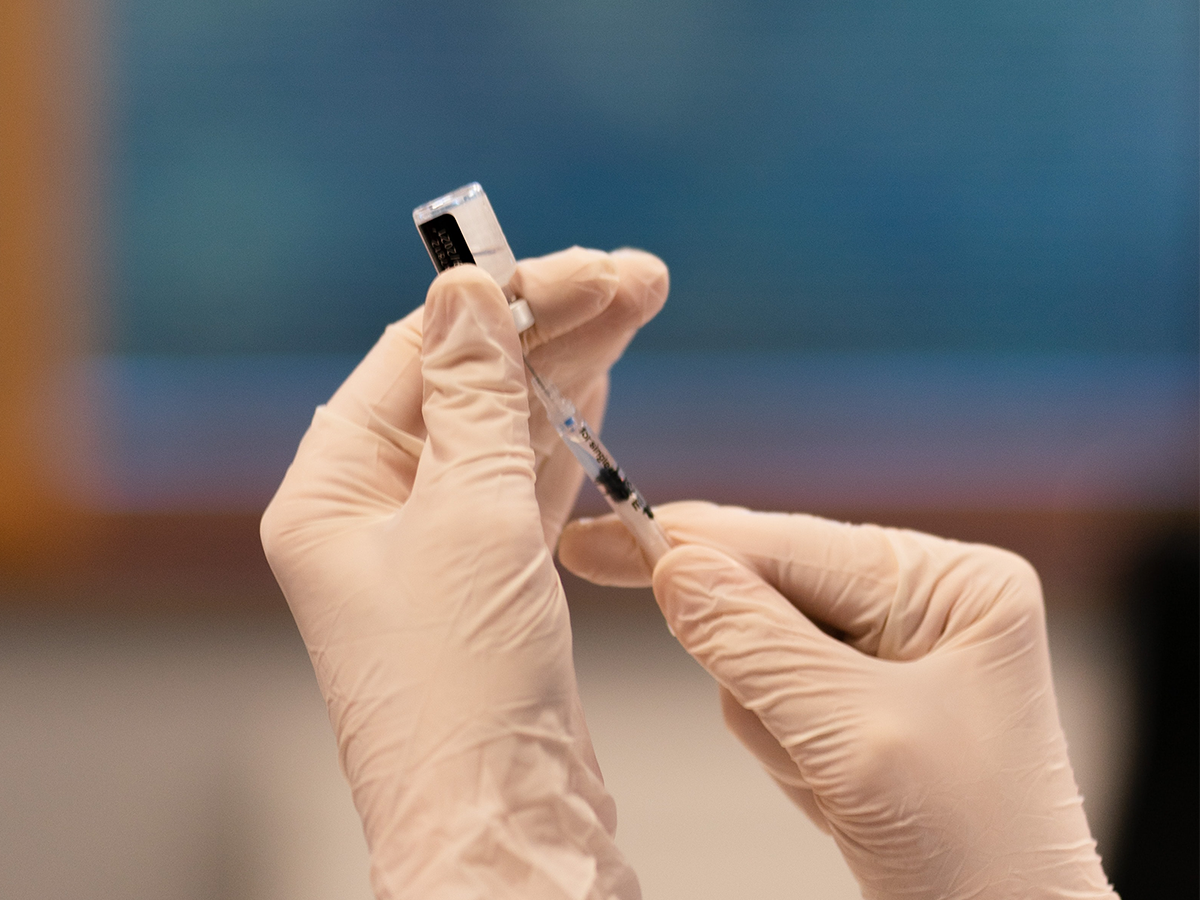
ICAP Grand Rounds Webinar — Long-Acting Injectable Cabotegravir for HIV Prevention: Promises and Pitfalls - ICAP at Columbia University
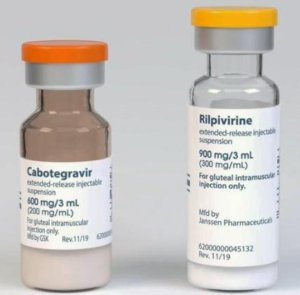
NICE approves long-acting cabotegravir and rilpivirine injections in England and Wales | HTB | HIV i-Base

HIV AIDS Treatment - Cabotegravir & Rilpivirine long acting injectable medicine for HIV patients - YouTube

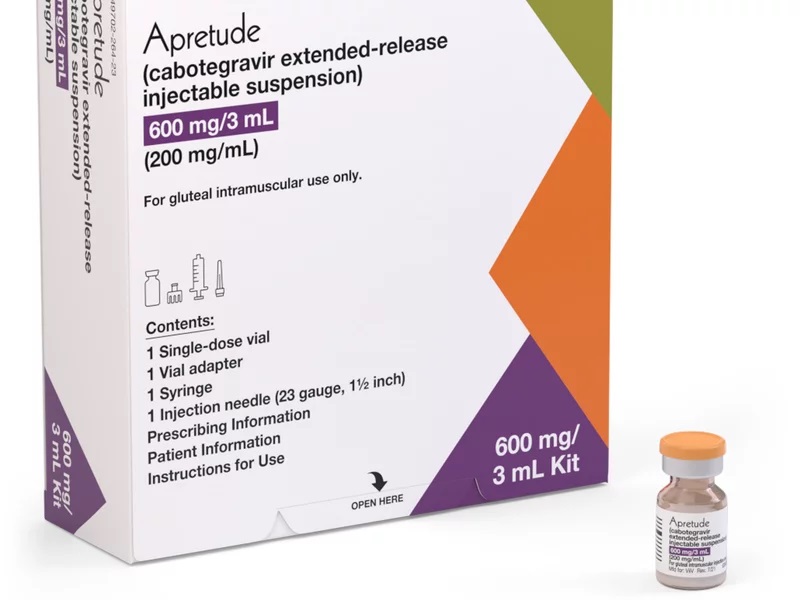
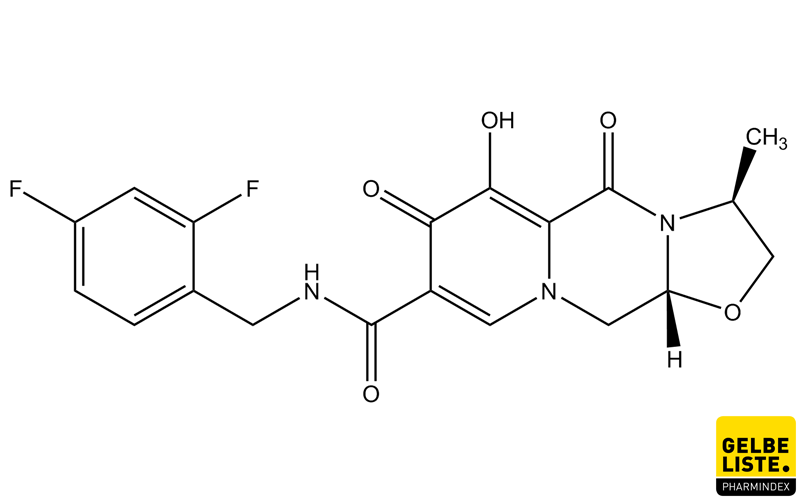
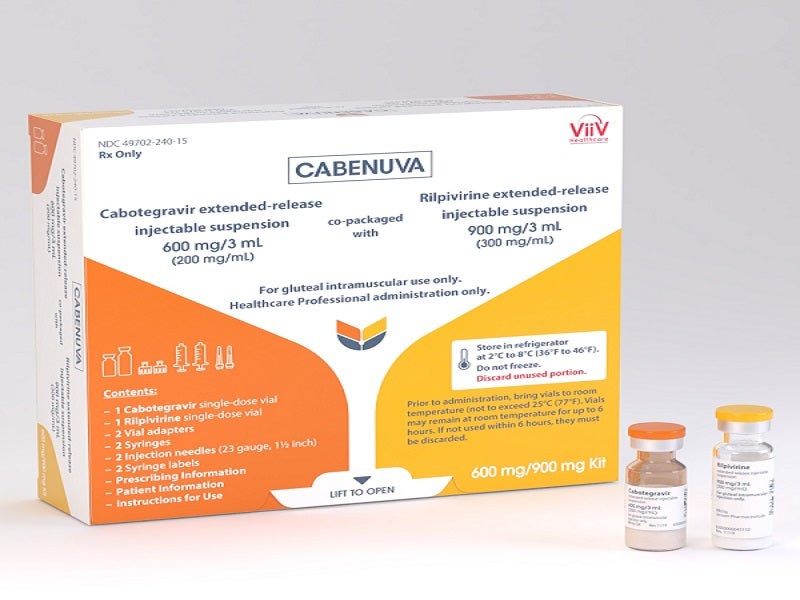
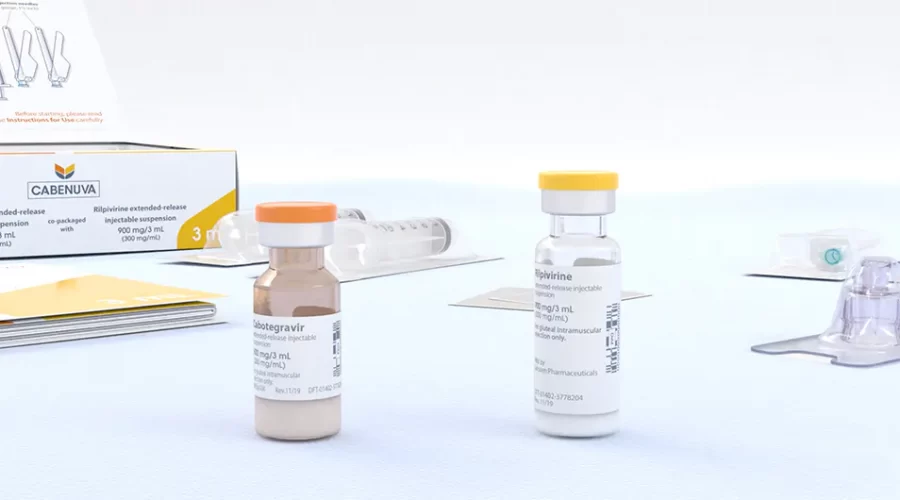
ViiV Healthcare announces US FDA approval of Apretude (cabotegravir extended-release injectable suspension), the first and only long-acting injectable option for HIV prevention | Business Wire

Predicted effects of the introduction of long-acting injectable cabotegravir pre-exposure prophylaxis in sub-Saharan Africa: a modelling study - The Lancet HIV

New formulations and alternative injection sites might allow self-administration of long-acting cabotegravir and rilpivirine | aidsmap