Genetic Counseling and Testing Programs*† for Hereditary ATTR (hATTR) Amyloidosis Offered at No Charge‡

Alnylam erhält positive CHMP-Stellungnahme zu Vutrisiran für die Behandlung der hereditären Transthyretin-vermittelten Amyloidose (hATTR) bei erwachsenen Patienten mit Polyneuropathie im Stadium 1 oder Stadium 2 | Business Wire

Alnylam Pharmaceuticals on Twitter: "Symptoms of acute hepatic #porphyrias are similar to those of other diseases, resulting in frequent misdiagnosis. Genetic testing through Alnylam Act may help: https://t.co/q9990gYwya https://t.co/oLYz6BU69U" / Twitter

ALNYLAM ACT Trademark of Alnylam Pharmaceuticals, Inc. - Registration Number 5498799 - Serial Number 87340411 :: Justia Trademarks

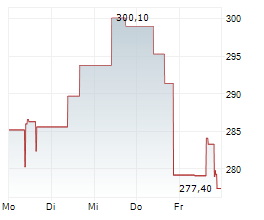
IRA Effect: Alnylam Acting 'Rationally' In Halting Second Orphan Indication For Amvuttra – Analysts :: Pink Sheet