China ACE 083 Serbuk RAW membekalkan kesucian tinggi, berkualiti tinggi ACE 083 Serbuk RAW membekalkan kesucian tinggi pada Bossgoo.com

Local administration of ACE-083 in wild-type mice causes dose-dependent... | Download Scientific Diagram

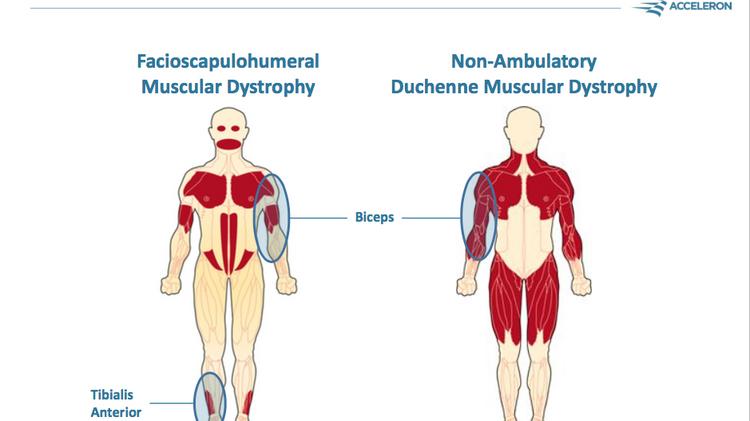
Acceleron Receives FDA Fast Track Designation for ACE-083 in Facioscapulohumeral Muscular Dystrophy (FSHD) - Drug Discovery and Development

Randomized phase 2 study of ACE‐083, a muscle‐promoting agent, in facioscapulohumeral muscular dystrophy - Statland - 2022 - Muscle & Nerve - Wiley Online Library

ACE-083 1000 mcg muscular dystrophy research - SuperhumanStore - longevity, dutasteride mesotherapy, NAD+, peptides, Cerebrolysin, exosomes, anti-aging, senolytics

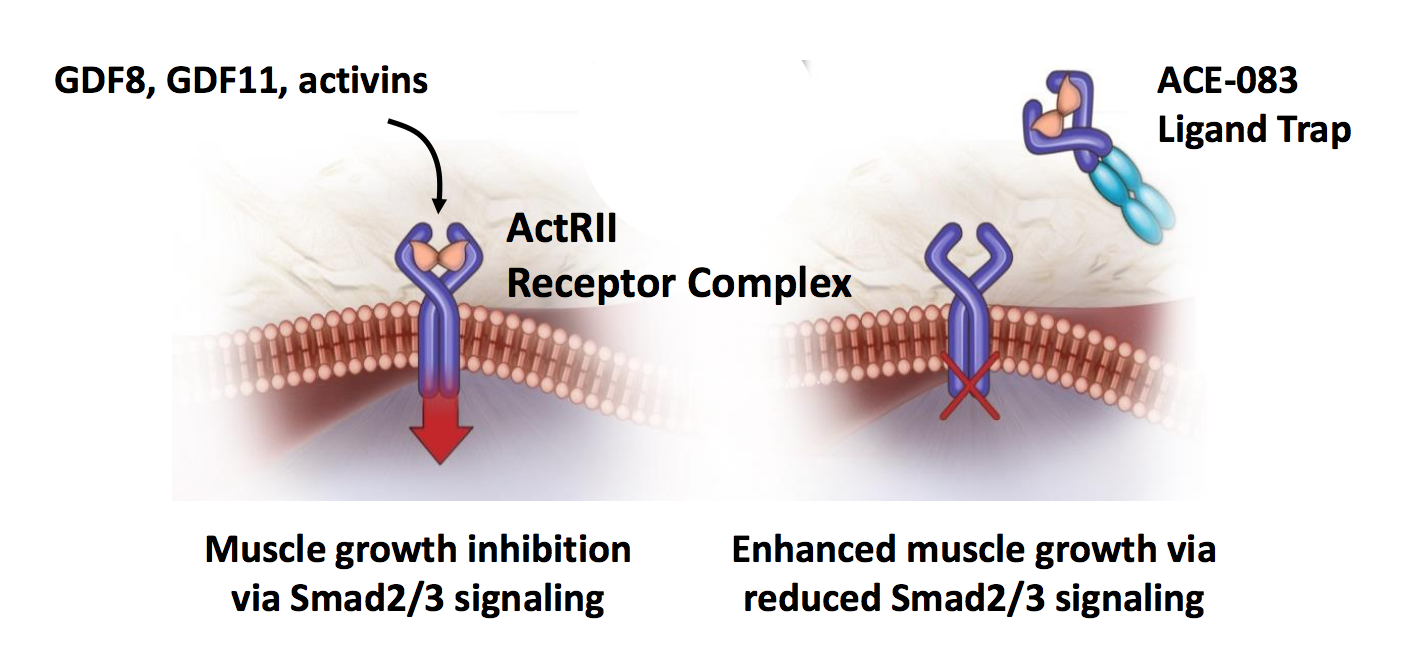
Locally acting ACE‐083 increases muscle volume in healthy volunteers - Glasser - 2018 - Muscle & Nerve - Wiley Online Library
FDA Grants Orphan Drug Designation to Acceleron Pharma's ACE-083 Muscle Growth Drug for Charcot-Marie-Tooth Disease - Quest | Muscular Dystrophy Association