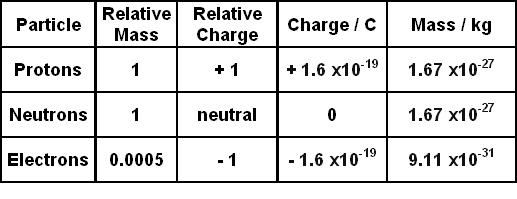
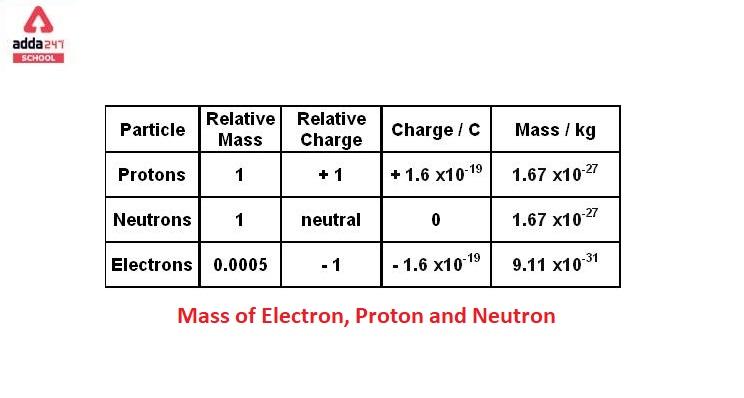
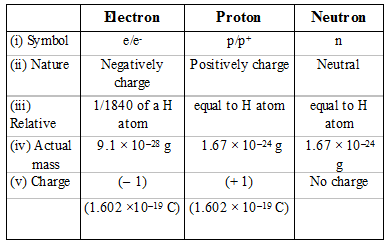
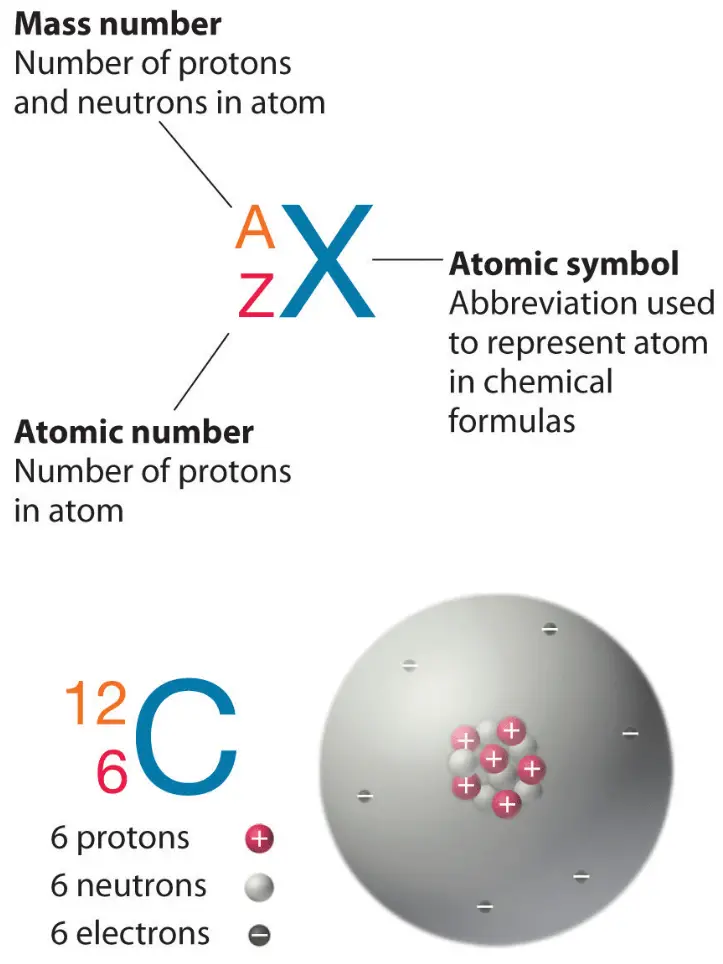
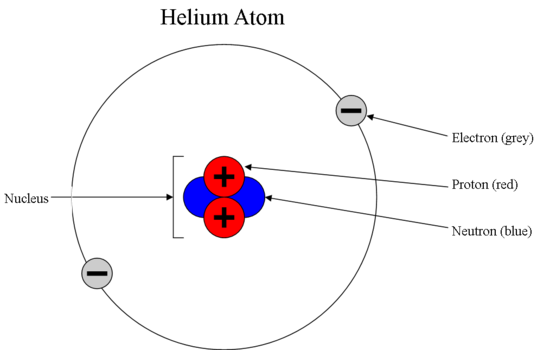
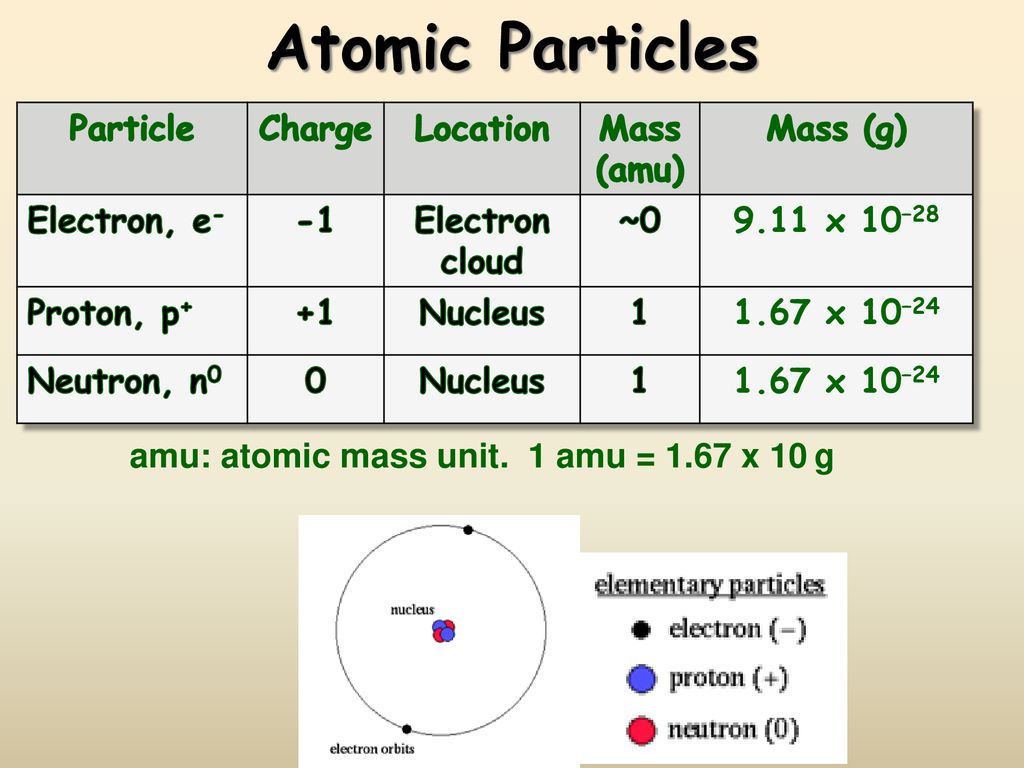
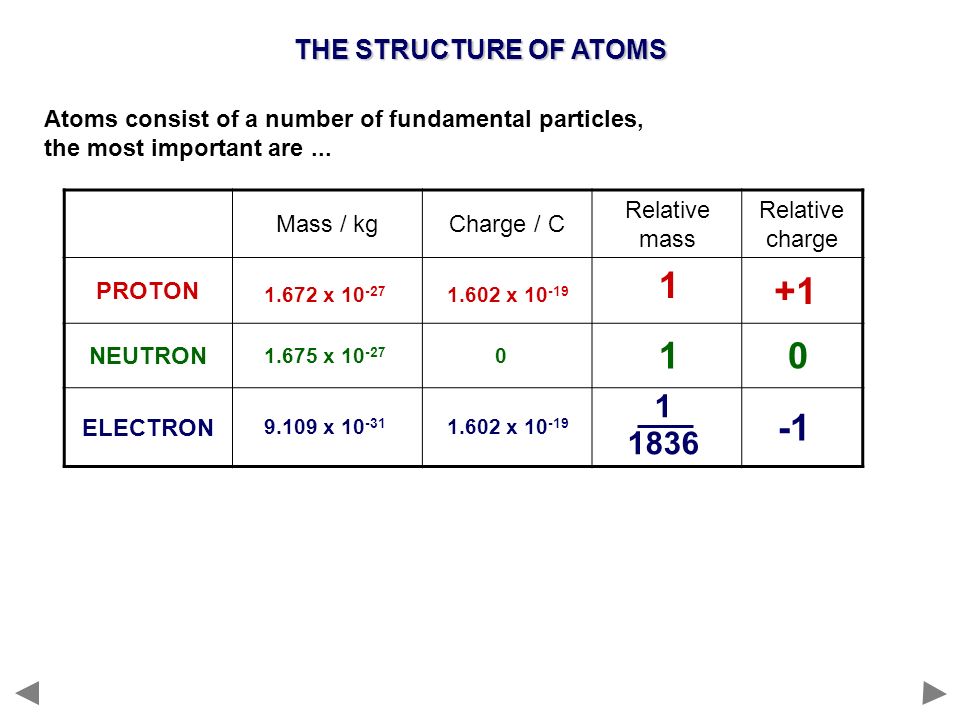
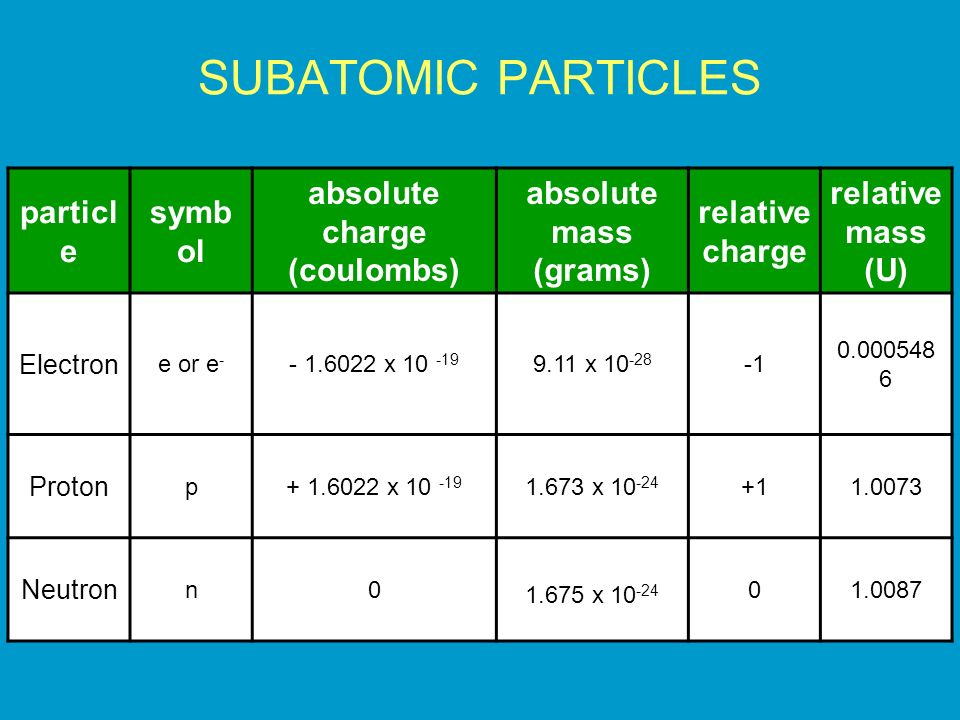
1.3 Understanding Atomic Mass. A Review: Subatomic particles Electron Proton Neutron NameSymbolCharge Relative mass Actual mass (g) e-e- p+p+ nono ppt download

Absolute charge/ relative charge/ mass in kg and u ( a.m.u) of electron/ proton & neutron Atm str. - YouTube

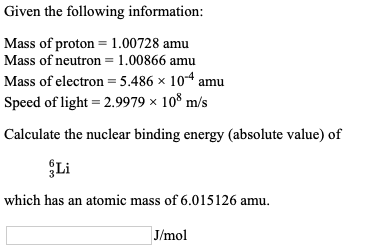
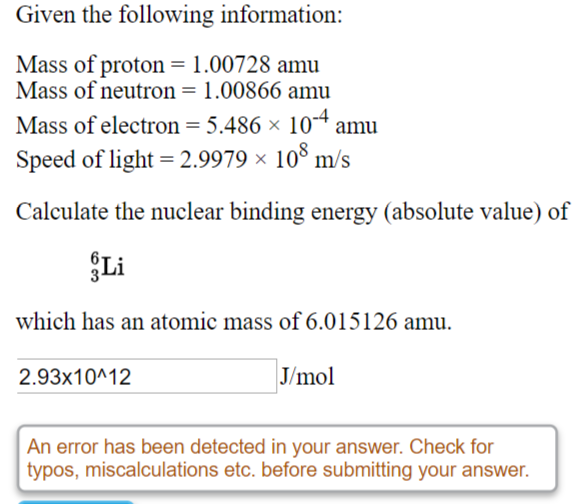
SOLVED: CHEMWORK Given the following information: Mass of proton 1.00728 amu Mass of neutron = 1.00866 amu Mass of electron = 5.486 x 10-4 amu Speed of light 2.9979 x 108 m/s

2.1.2 State the relative masses and charges of protons, neutrons and electrons IB Chemistry - YouTube

ATOMS AND ATOMIC STRUCTURE 1.Introduction: definition, constituents & Thomson's model of an atom 2. Rutherford's α -scattering experiment 3. Rutherford's. - ppt download