Generic Ethyl Alcohol aka Ethanol,absolute, 99.99% pure 500 ml CAS # 64-17-5, Pack of 20 : Amazon.in: Industrial & Scientific
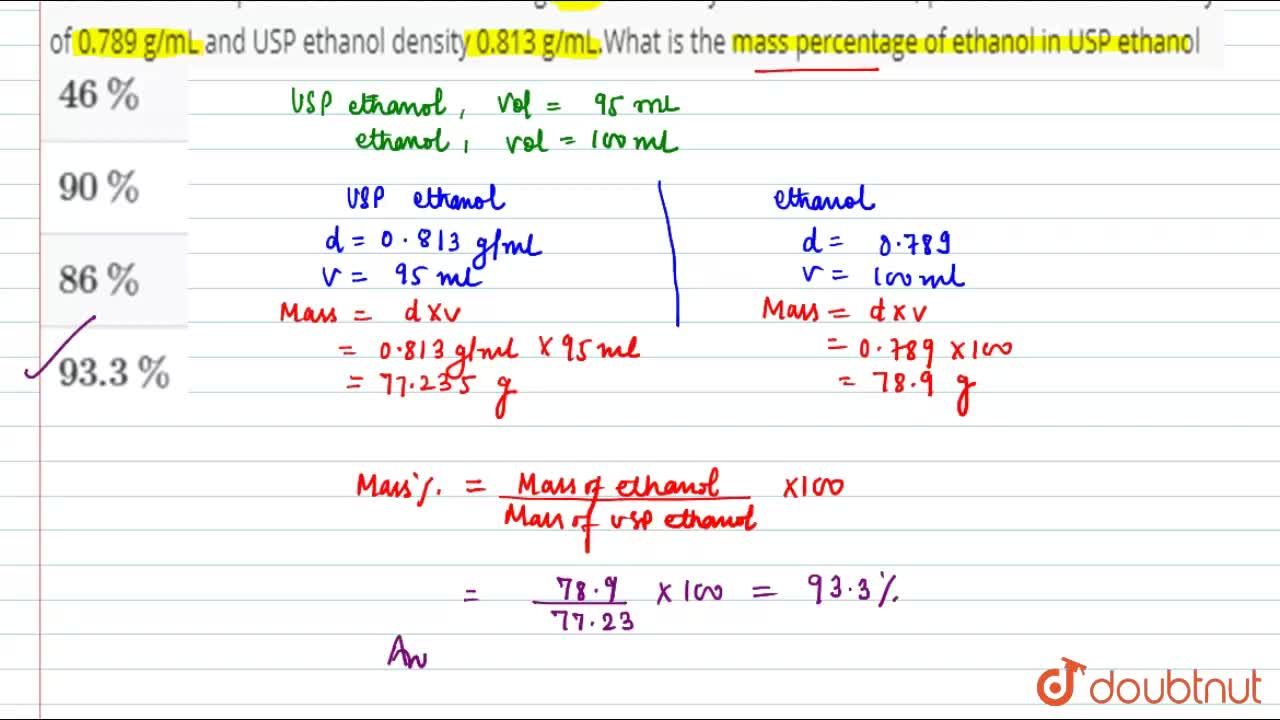
USP ethanol in aqueous solution is containing 95% ethanol by volume. At 20^(@)C, pure ethanol has a density of 0.789 g/mL and USP ethanol density 0.813 g/mL. What is the mass percentage
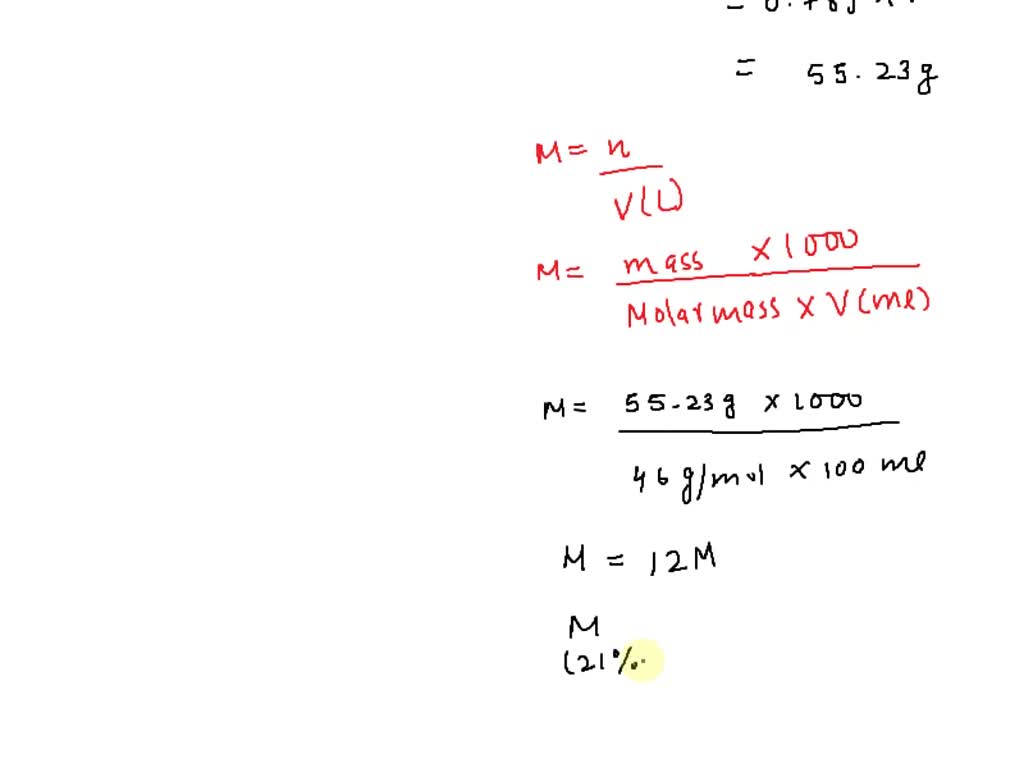
SOLVED: A stock solution of 70% v/v ethanol was diluted with distilled water to prepare 1L of 21% v/v ethanol. (Density of ethanol = 0.789 g/mL; MW of ethanol = 46 g/mol)
![Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 .[Assume the density of water to be one] Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 .[Assume the density of water to be one]](https://i.ytimg.com/vi/Jdkez2jpR0s/maxresdefault.jpg)
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 .[Assume the density of water to be one]

100 mL of ethyl alcohol (d = 0.92 g/mL) and 900 mL of water (d = 1 g/ml) are mixed. The molarity and molality of the resulting solution are:
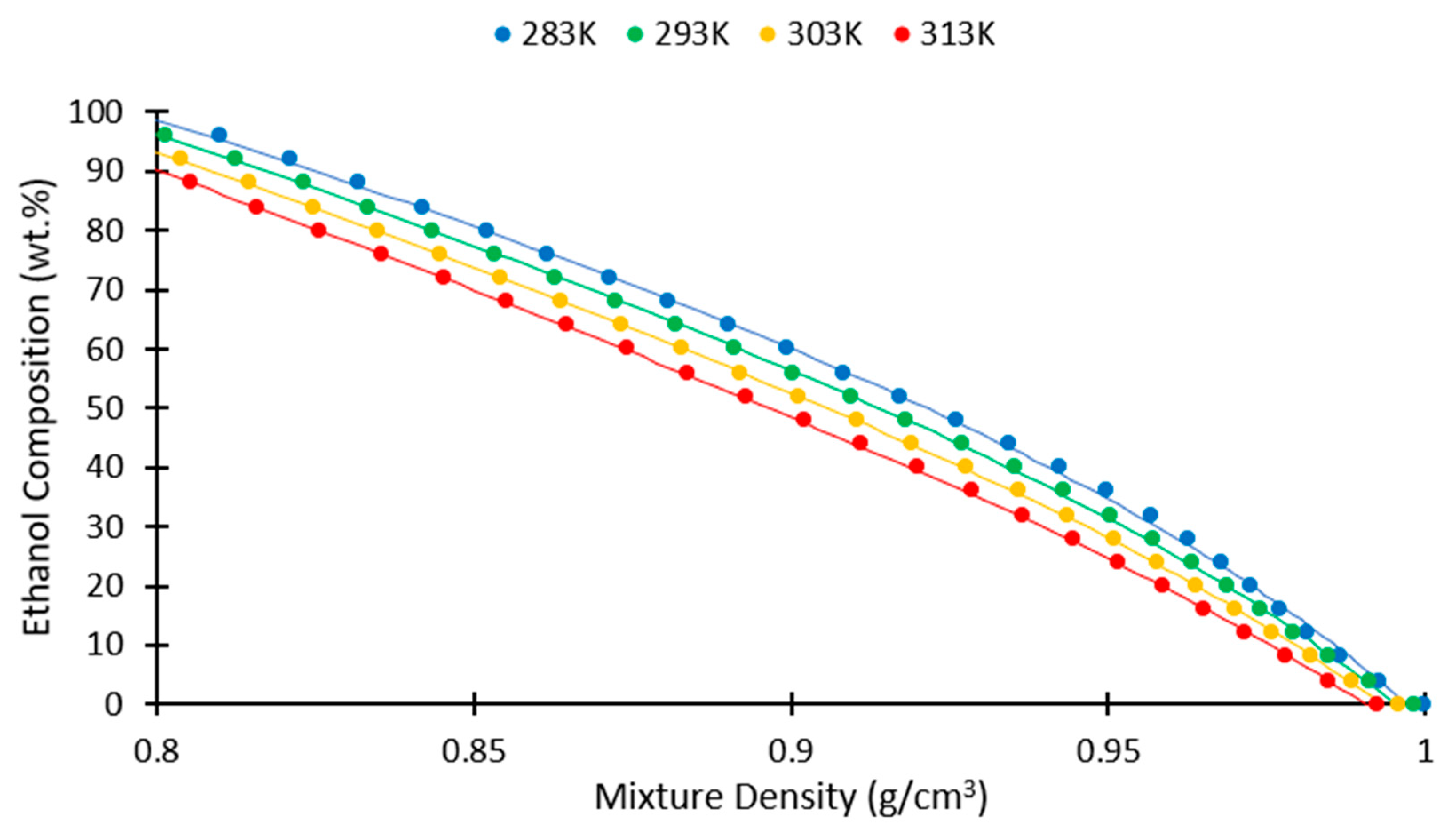
Fermentation | Free Full-Text | Computing the Composition of Ethanol-Water Mixtures Based on Experimental Density and Temperature Measurements

Generic Ethyl Alcohol aka Ethanol,absolute, 99.99% pure 500 ml CAS # 64-17-5, Pack of 20 : Amazon.in: Industrial & Scientific
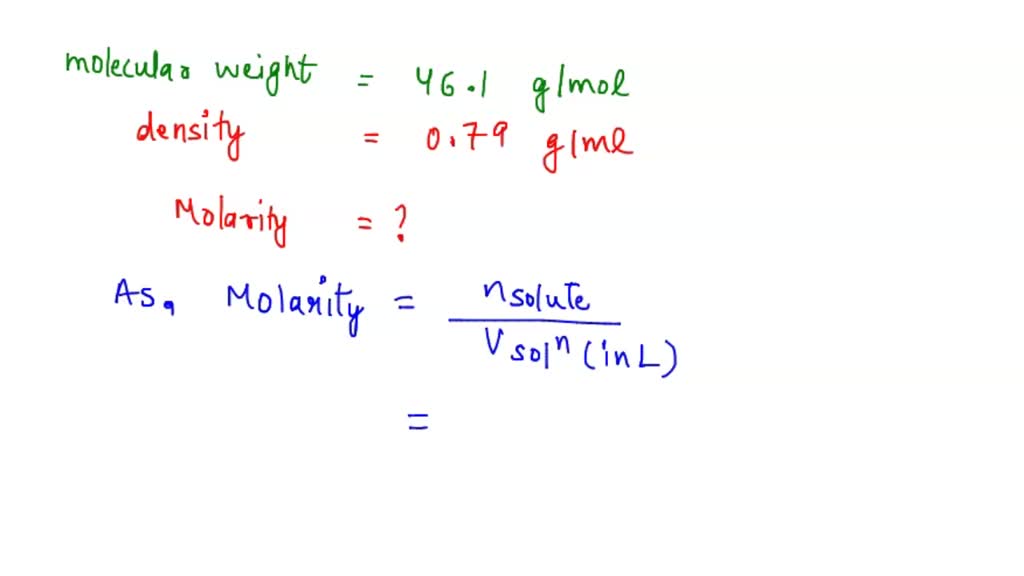
SOLVED: Question 1 The molecular weight of ethanol (C2H5OH) is 46.1 and the density of absolute (100%) ethanol = 0.79 g/mL a) Calculate the molarity of absolute ethanol (100% ethanol). b) The

SOLVED: A.) Calculate the error if the measured density value of ethanol is 0.802 g/mL and the true value is 0.789 g/mL. B.) Calculate the error if the mass of a gold